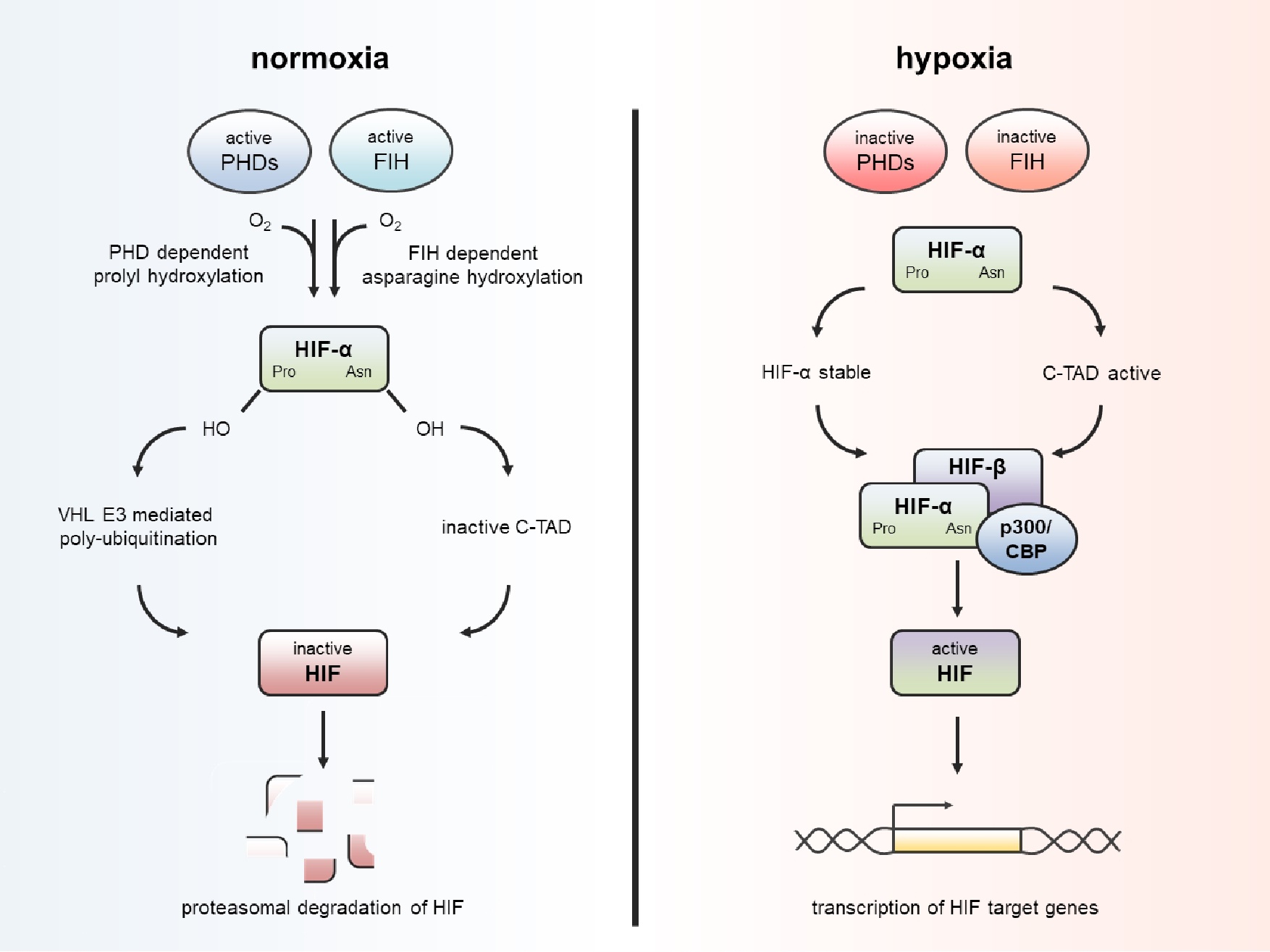
Fig. 1. If sufficient O2 is present (normoxia), the proline (Pro) and asparagine (Asn) residues of hypoxia-inducible factor (HIF)-α subunits are hydroxylated by prolyl hydroxylases (PHDs) and factor-inhibiting HIF (FIH). This leads to recognition by von Hippel-Lindau protein (pVHL), polyubiquitination and subsequent proteasomal degradation; in parallel, hydroxylation of the C-terminus of HIF-α prevents the binding of co-activators for transcription. With lack of oxygen (hypoxia) the hydroxylases are reduced in activity, resulting in accumulation and nuclear translocation of HIF-α and subsequent dimerization with HIF-β in the nucleus. The HIF complex in the nucleus recruits the co-activator p300/CBP and enables the transcription of HIF target genes.